Our immune system is a vast and powerful network, filled with specialized cells and proteins designed to protect us from infections and diseases. However, in autoimmune diseases like MS, some of these specialized immune cells misbehave.
Recently, a treatment called Chimeric Antigen Receptor (CAR) T-cell therapy, initially developed for cancer, has shown potential for treating autoimmune diseases as well, and many clinical research studies are underway to learn more about the safety and efficacy of this novel approach. Below is an overview of CAR T-cell therapy, key questions that are being studied, and future possibilities in conditions like MS.
The Immune System, Autoimmunity, and MS
The immune system consists of trillions of cells working together to defend us. Some cells act as “first responders,” quickly moving to infection sites and neutralizing threats of illness and disease. Others, like T-cells and B-cells, serve as “special forces,” each tailored to recognize specific threats. When they detect an invader, T-cells and B-cells act swiftly, with B-cells also producing antibodies that target pathogens.
However, in autoimmune diseases, these “special forces” can mistakenly attack the body’s own tissues, leading to chronic and sometimes debilitating health conditions. To prevent this, immune cells undergo rigorous testing in the body before being released. Even so, autoreactive harmful cells can slip through and contribute to autoimmune disease.
Learn more about CAR T-cell Therapies in Dr. Amanda Piquet’s recent Education Summit presentation. (YouTube)
MS affects the central nervous system, including the brain and spinal cord, and is triggered by an overactive immune response. In MS, the immune system mistakenly targets normal cells and tissues in the central nervous system, treating them as foreign invaders. This autoimmune response leads to inflammation and damage. Immune cells attack or signal damage to the protective coating around nerve fibers, called myelin, the cells responsible for producing myelin, and the nerve fibers themselves. Over time, this damage accumulates, leading to disease progression and worsening symptoms.
Current MS Treatments and Unmet Needs
MS currently has more than 20 approved disease modifying therapies (DMTs) aimed at regulating or suppressing specific immune system processes to reduce inflammation, minimize damage, and slow the progression of disability. Different classes of DMTs target the immune system in various ways, all with the shared goal of decreasing MS disease activity. While current DMTs are effective for many, they do not work for everyone—particularly for individuals with secondary progressive MS or primary progressive MS. MS may continue to worsen over time, even while on treatment. Researchers are actively exploring new therapeutic targets and developing innovative approaches to improve MS management.
Most of these current disease modifying therapies work outside the central nervous system (CNS) in the blood and lymph nodes. These therapies have a difficult time effectively getting into the CNS because they are very large molecules, leaving resident immune cells in the brain and spinal cord untouched. This is a significant challenge, as immune cells within the CNS may drive progression and long-term disability in MS.
CAR T-cell therapy offers hope of penetrating the CNS and targeting these cells, which could help prevent disease progression in MS and other neuroinflammatory conditions.
CAR T-Cell Therapy: A Game-Changer for Cancer and Autoimmune Disease?
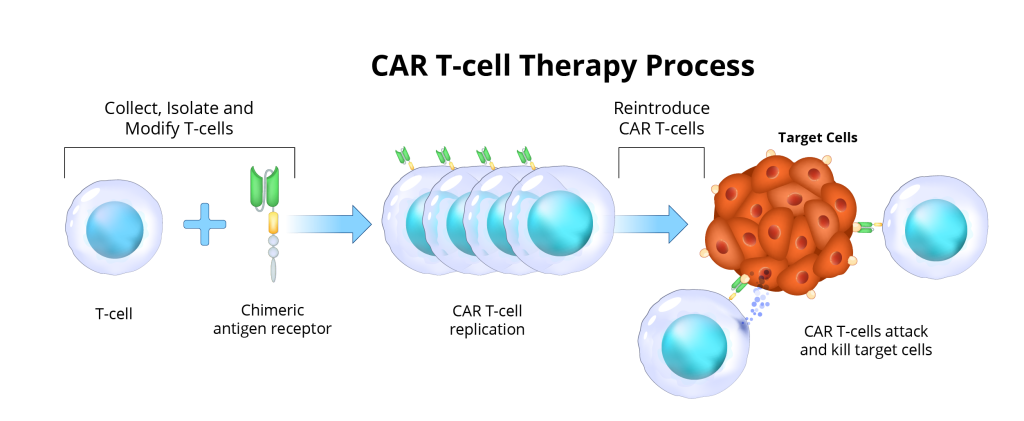
CAR T-cell therapy has already changed cancer treatment, especially in some blood cancers. In CAR T-cell therapy, T-cells from a patient’s blood are engineered in a lab to recognize and attack specific cells. After being reintroduced into the patient’s body, these modified T-cells target and eliminate harmful cells. Now, scientists are investigating this approach for autoimmune diseases, designing CAR T-cells to seek out and neutralize cells responsible for autoimmune attacks.
Why CAR T-Cell Therapy May Hold Promise for MS:
- B-Cell Involvement: Research shows that B-cells play a significant role in MS. While current B-cell-depleting therapies work in the bloodstream, they struggle to reach B-cells within the CNS. CAR T-cells could be a potential solution.
- Ability to Reach the Brain: Unlike some therapies, CAR T-cells have a greater chance of crossing the blood-brain barrier, allowing them to act on immune cells in the CNS.
- Unmet Needs in MS Treatment: Though current MS treatments help many, they may not prevent long-term disability in all patients, particularly in progressive stages of the disease. The greatest unmet need in MS treatment remains in progressive MS.
 How CAR T-Cell Therapy Works
How CAR T-Cell Therapy Works
CAR T-cell therapy begins by collecting a patient’s immune cells and taking them to the lab to isolate the T-cells. Then they will undergo a process to modify the T-cells by putting the CAR protein on the top of the T-cells to program them to target the misbehaving B-cells that are causing disease. For most studies that are ongoing for MS, the CAR T-cells are targeting CD-19 which is one key protein marker on B-cells.
Once the CAR T-cells are made in the lab, patients undergo lymphodepletion, which is a chemotherapy regimen over a couple of days to prime the immune system to ready the body to receive those T-cells back. Then patients are admitted to the hospital to receive their CAR T-cells through an infusion. Once reintroduced, these CAR T-cells seek out and attack their target, ideally providing a one-time, long-lasting treatment by eliminating those misbehaving B-cells completely.
Safety Concerns and Considerations
While CAR T-cell therapy offers potential promise, it comes with risks. Acute side effects may include reactions during the blood collection or side effects of chemotherapy. However, more serious risks, such as cytokine release syndrome (CRS) and neurotoxicity, could occur after the T-cell infusion. Long-term risks, like infections and immune-related issues, are also being evaluated.
The CAR-T study protocols are rigorous and require study participants to be admitted to the hospital typically for 7-10 days for the infusion preparation and infusion process, followed by frequent post-infusion monitoring for at least one month, requiring patients to stay within 30-60 minutes of the hospital.
Despite these risks, CAR T-cell therapy could ultimately reduce the need for ongoing treatments, as it is designed to work over the long term with a single application. Several studies are underway to explore the safety and effectiveness of CAR T-cell therapy in MS and many key unanswered questions remain.
The Future of CAR T-Cell Therapy in Autoimmune Disease Including MS
Although CAR T-cell therapy is still in the experimental phase for autoimmune diseases including MS and much more research is needed, it holds significant potential. Clinical trials are underway to understand its effectiveness and safety in conditions like MS, stiff person syndrome, and other rheumatologic disorders. While much research is needed, if successful, CAR T-cell therapy may offer a new approach for individuals living with progressive forms of MS.
Newer approaches are examining using cells that are already prepared (allogeneic or “off the shelf”) that would bypass the step of having to purify cells from each person, and other approaches are looking at using types of cells other than T-cells such as Natural Killer (NK) cells to make CAR-NK cells.
For a current list of CAR T-cell studies underway for MS, visit the National Institute of Health at ClinicalTrials.gov. For information regarding the CAR T-cell study in Stiff Person Syndrome, please visit TinyUrl.com/InforMS-SPS.