Proposed Changes to How We Determine Who Has MS
At the ECTRIMS meeting in September, there was a presentation on proposed updates to the 2017 McDonald criteria to diagnose MS. It’s important to note the word “proposed” as these revisions are not final and may still undergo changes before they are peer reviewed and finalized. For that reason, this article is not meant to be a blueprint on how to diagnose MS with the new criteria. It’s an overview of interesting changes and concepts meant to help you get familiar with them before the final version is released.
The McDonald Criteria
The McDonald Criteria were first published in 2001 as a way for neurologists to standardize the conditions that must be met for a diagnosis of MS. This first version added MRI into the criteria for MS. Before MRI was used in 1984, patients could tell doctors what they were experiencing, and doctors could observe if someone’s symptoms worsened in a hot bath. Spinal fluid markers such as oligoclonal bands were the only biomarker available to diagnose MS, and these are not specific for MS. Many doctors weren’t keen to give a diagnosis of MS quickly because there were no treatments available. When treatments became available, more doctors were willing to diagnose MS but it was still a lengthy and somewhat subjective process. However, as our technology has improved and as we have learned more about MS, the need for standardization has increased. The McDonald criteria have been revisited and revised over time. This will not be the last revision as there is still lots to learn about MS.
The goal of the latest revisions is to decrease the amount of time it takes to get diagnosed with MS while also minimizing the risk of misdiagnosing MS. We know that it can take years for someone to be diagnosed with MS, and we also know that the quicker one gets a diagnosis and is able to access a disease modifying treatment, the better chance they have to minimize future disease progression. A commonly cited misdiagnosis rate is 20%. This can lead to a delay in access to the right medications, decreased quality of life due to symptoms, and emotional distress. In order to address these issues, the revised criteria need to increase sensitivity and specificity.
Sensitivity and Specificity
“Sensitivity” refers to the ability of a test to correctly identify individuals who have a disease, minimizing false negatives, while “specificity” refers to the ability of a test to correctly identify individuals who do not have a disease, minimizing false positives. It is a challenge to increase both of these at once because as sensitivity increases, specificity tends to decrease, and vice versa. The revisions to the criteria take both sensitivity and specificity into consideration as part of the bigger picture of diagnosing MS.
Dissemination in Space
In the 2017 McDonald Criteria, you needed to have “dissemination in space” which means that you had to have one or more MS-typical T2 lesions in two or more areas of the central nervous system (CNS): periventricular, cortical/juxtacortical, infratentorial regions of the brain, or spinal cord. Periventricular refers to the area around the ventricles, which are the fluid-filled spaces in the brain that contain cerebrospinal fluid. The periventricular area is where nerve fibers carry messages from the brain to the body’s muscles. Cortical refers to lesions that occur within the cerebral cortex, the outermost layer of the brain. Juxtacortical lesions are located at the border of the gray matter and subcortical white matter. The infratentorial regions of the brain include the brainstem and cerebellum. The spinal cord and central nervous system go through the cervical and thoracic spine, ending just below the rib cage at about the first or second lumbar vertebrae.
The proposed revisions will add a fifth area: the optic nerve. Inflammation of the optic nerve is called optic neuritis. It can result in vision loss (usually in one eye), ocular pain, or a frontal headache. Approximately 20-35% of people present with optic neuritis as their first MS symptom and approximately 50% of people living with MS will experience it at some point. Within the Hispanic population, individuals with MS are more likely to present with optic neuritis compared to other ethnicities, often experiencing it at a younger age and with greater severity than non-Hispanic populations. Studies have shown that Hispanics with MS can be up to twice as likely to present with optic neuritis compared to white individuals with MS. Adding the optic nerve as a 5th area to prove dissemination in space will help identify MS earlier in a diverse population.
Although optic neuritis can be seen on an MRI, it can also be detected during a visual evoked potential (VEP) test or optical coherence tomography (OCT) test. A VEP test is a non-invasive procedure that measures electrical activity in the visual pathways of the brain. It’s used to assess the function of the eyes, optic nerves, and other parts of the visual system. An OCT test is a non-invasive imaging technique that creates cross-sectional images of the eye. A clinician will also need to rule out any other possible causes of optic neuritis as it is not specific to MS.
Another change to the dissemination of space requirement is that in patients with progressive disease, two spinal cord lesions will meet the requirement, they do not need lesions in other areas of the CNS.
Dissemination in Time
The revised criteria will take out the requirement of dissemination in time. It was originally there because we know that MS is a chronic condition and you had to prove that you met this to be diagnosed. However, we now know that most people with either clinically isolated syndrome, CIS, (one exacerbation), or radiologically isolated syndrome, RIS, (one lesion found on MRI without symptoms), will go on to develop MS, and we have seen in trials that there is a benefit to treating patients before their second attack or lesion.
Kappa Free Light Chains
In the 2017 criteria, you could meet the dissemination in time requirement through one exacerbation or lesion and the presence of oligoclonal bands (OCB). OCB are made up of antibodies which indicate inflammation in the central nervous system. It takes time to develop OCB, which would prove that a person has had MS for some time. However, it can be difficult to measure OCB. Another biomarker that is easier to quantify and will be added to the revised criteria is kappa free light chains (KFLC). KFLCs are a product of B-lymphocytes that are produced when your body makes antibodies and are elevated in people living with MS. In the US, we are more likely to measure OCB during diagnosis, but in other parts of the world, the addition of KFLCs may be helpful to diagnose MS easier.
Central Vein Sign (CVS) Lesion
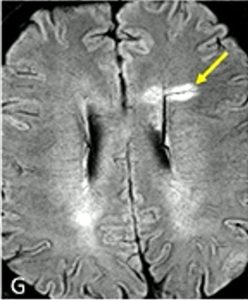 A concept that may be new to patients is the central vein sign (CVS) lesion. With the higher power MRIs that are available in research today, the 7T machine, we can see if a lesion has developed around a vein running through the middle. Hence the name, central vein sign. More than 50% of patients with MS will have this type of lesion.
A concept that may be new to patients is the central vein sign (CVS) lesion. With the higher power MRIs that are available in research today, the 7T machine, we can see if a lesion has developed around a vein running through the middle. Hence the name, central vein sign. More than 50% of patients with MS will have this type of lesion.
Once these were identified on the stronger MRI machines, a protocol was developed to help see them in lower power machines used in clinical care. If a patient has 6 or more CVS lesions (called select 6), that can be used as one more vote toward confirmation of MS. This is especially helpful in patients who may have lesions due to other causes such as vascular conditions because those lesions do not form around a vein in this way. CVS lesions are not required to make a diagnosis of MS, they can only help confirm it. Most clinical MRIs don’t quantify CVS yet, but it may become more common as these new criteria become accepted.
Paramagnetic Rim Lesion (PRL)
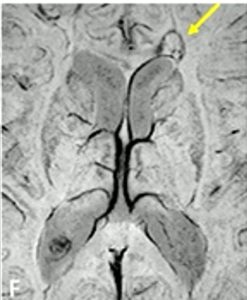 Another term that may be unfamiliar is paramagnetic rim lesions, or PRLs (pronounced “pearls”). PRLs are a type of lesion specific to MS. Also known as “chronic active lesions,” PRLs feature a central core of demyelination surrounded by persistently inflamed cells, typically microglia. These microglia may contribute to the gradual expansion of the lesion. They also contain iron, which appears as a rim around the lesion on the same MRI sequence that is used to detect the central vein sign.
Another term that may be unfamiliar is paramagnetic rim lesions, or PRLs (pronounced “pearls”). PRLs are a type of lesion specific to MS. Also known as “chronic active lesions,” PRLs feature a central core of demyelination surrounded by persistently inflamed cells, typically microglia. These microglia may contribute to the gradual expansion of the lesion. They also contain iron, which appears as a rim around the lesion on the same MRI sequence that is used to detect the central vein sign.
Radiologically Isolated Syndrome
A major change that will be possible with these revisions is that someone can be diagnosed with MS without ever experiencing MS symptoms. Before, if someone went in for an MRI for a reason unrelated to MS and lesions that looked like MS were found, they would be told that they had radiologically isolated syndrome (RIS) and that it may develop into MS but treatment likely wasn’t recommended until they met the criteria. With recent research, we know that many people with RIS will develop MS and that there is benefit to treating it before clinical symptoms manifest. These revised criteria allow for that diagnosis and access to treatment to happen faster.
Conclusion
What will not change is that MS is a disease of rule-out. You must eliminate the possibility of other likely diagnoses. These new recommendations also do not limit who has been previously diagnosed with MS, they will not “un-diagnose” anyone. There are some special considerations for diagnosing specific populations like pediatric patients and those over age 50 that we did not cover in this article. Once the criteria are peer-reviewed, published, and final, we’ll share all of the revisions in another update. The hope is that these revised criteria will allow for patients to be diagnosed quicker and with a lower misdiagnosis rate. The wider variety of tests that may be used to fulfill the criteria will benefit those who do not have access to the more stringent tests required before. For more information, please watch the presentation “ECTRIMS Updates” on our YouTube channel and keep an eye out for our announcement when the criteria are finalized. n






