
It’s often a long, stressful road to a diagnosis of multiple sclerosis. While technology and medical techniques have certainly advanced in recent years, the diagnosis process still involves collecting a lot of information and putting those pieces together to get the big picture.
Despite the many advancements in our understanding of MS, there’s still no single symptom, or clinical observation, or laboratory test that can determine if you have it. Early symptoms, initial doctor visits, and observations and tests are all pieces of the puzzle that have to be put together.
How we collect and use that information has changed, and in most cases that’s made diagnosing MS a faster and more accurate process. But the reality is that the brain and central nervous system are complicated things, and there are countless possible causes for various neurological symptoms. When we diagnose MS, we’re not only looking for signs of the disease itself, but we’re also looking to rule out a host of other conditions.
In this issue we’ll go through a typical path of diagnosis, from the earliest symptoms to the final determination. We’ll also look at what neurologists are looking for, how they find it, and the guidelines they use for putting it all together — and how that’s changed over time. Finally, we’ll see what’s on the horizon and how research at the Rocky Mountain MS Center at CU may someday change the way we diagnose MS.
Finding a ‘Typical’ Presentation in a Disease that’s Anything But Typical
If you’re reading this, you or a loved one probably have direct experience with receiving an MS diagnosis.
And you probably know that diagnosing MS isn’t easy. There’s no one “typical” presentation, or normal set of symptoms that indicate with certainty that someone has MS. In this article we’ll illustrate a typical diagnosis process — to the extent that we can — understanding this won’t be a perfect description of anyone’s personal experience.
Most people start their diagnosis journey at the first onset of symptoms, by visiting their primary care physician or, if the symptoms are severe enough, a local emergency room.
“Numbness, weakness, clumsiness usually on particular parts of the body like one arm or one leg,” are some of the most common first symptoms people notice, according to Dr. Robert Gross of the RMMSC at CU. “Something that would suggest it’s affecting a focal part of the nervous system, as opposed to feeling generally weak which could be due to a variety of non-neurologic processes like dehydration.”
“Vision is often affected; loss of vision or blurry vision in one eye, which might suggest an optic neuritis, or double vision,” said Gross. “Or slurred speech, balance problems, bladder dysfunction — these are all common symptoms that someone might experience during a relapse or exacerbation.”
Taken by themselves, these symptoms don’t give us a clear path toward MS. Especially if this is your first time with these symptoms, and you’re seeing your PCP or an ER doctor who may not have experience with MS, the next step is most likely referral to a neurologist.
“There are potentially many conditions that might cause symptoms similar to those seen in MS,” says Dr. John Corboy, Co-Director of the RMMSC at CU. “When we do a diagnostic work-up we’re trying to put the symptoms into context. We’re looking for neurological abnormalities in the examination that might explain the symptoms.”
Neurologists can take the evidence presented by the outward symptoms of MS, and start looking beneath the surface to determine what’s really going on.
“Patient history, neurological examination, MRI scans, lab tests, and to a lesser extent, spinal fluid analysis are the most common tools we use,” says Corboy.
The results of these exams and tests are the puzzle pieces used to put together a diagnosis of MS.
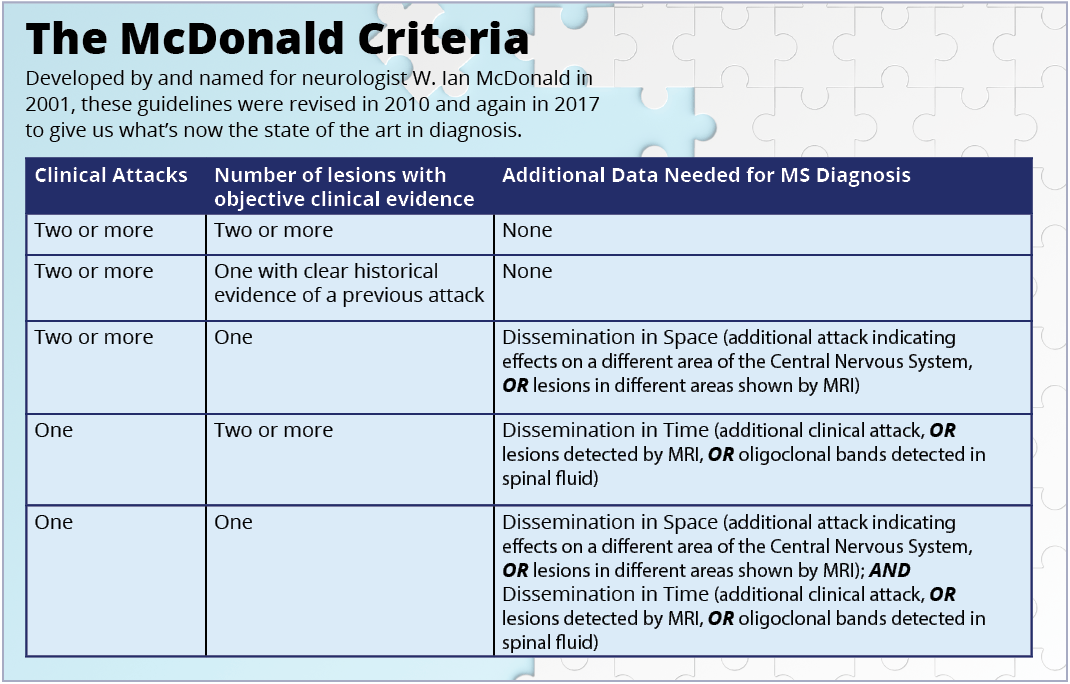
Neurologists use a series of guidelines known as the McDonald Criteria to help them make a final determination. Developed by and named for neurologist W. Ian McDonald in 2001, these guidelines were revised in 2010 and again in 2017 to give us what’s now the state of the art in diagnosis.
 “MS is still basically a clinical diagnosis,” says Gross. “In other words, we don’t have any truly reliable biomarkers that can say, ‘Yes, this person has MS.’”
“MS is still basically a clinical diagnosis,” says Gross. “In other words, we don’t have any truly reliable biomarkers that can say, ‘Yes, this person has MS.’”
“When a patient experiences a relapse or progression, that has to be evaluated by a neurologist to see if it’s consistent with MS,” said Gross.
For example, on an MRI, we can see lesions on the brain that show up as white spots. Different conditions can also produce such spots, but a well-trained eye can determine if what they’re seeing is likely to be lesions caused by MS.
A lumbar puncture is a procedure where spinal fluid is withdrawn from a patient, which is then examined to look for the presence of certain antibodies. If those antibodies are visible as “oligoclonal bands” on the lab report, that’s another indicator we’re looking at a patient with MS.
These results, along with physical exams, patient history and other information, can be put together using the McDonald Criteria.
“MS is an affirmative diagnosis, where MS criteria are met, and alternative diagnoses are excluded,” says Corboy. “Ultimately, the idea is that the individual would need to show ‘dissemination of lesions in time and space’ by a combination of clinical and laboratory signs.”
Dissemination in Time (DIT) and Dissemination in Space (DIS) are key concepts, and both are necessary for an MS diagnosis. Together, they show that MS activity is happening over a period of time, and in different parts of the nervous system.
Recent revisions to the McDonald Criteria have changed how we determine what constitutes both DIT and DIS.
Previously, time was calculated only by observing outward signs of exacerbations or by seeing active lesions on an MRI. Today when we see signs of older, inactive lesions on an MRI, we can use that to determine MS activity has been ongoing.
Similarly, space was previously calculated by having MS activity in at least two out of four regions of the nervous system (periventricular, juxtacortical, or infratentorial regions of the brain, plus the spinal cord). The latest revision added consideration of cortical lesions, which we now know to be common in MS, as a determining factor in DIS.
Prior to these revisions, there was a lot of waiting. For example, people who had a single exacerbation with no other evidence of MS were thought to have Clinically-isolated Syndrome (CIS); before they could start an MS treatment, they had to wait for a second attack to meet the DIT criteria.
“A lot of people who previously would have been identified as having CIS,” says Gross, “would now be classified as having relapsing remitting MS.”
Collecting all this evidence, and using the McDonald Criteria, has brought us to a place where MS diagnosis is faster and more accurate than ever.
Getting that diagnosis means they can start an MS treatment, and as you’ll see further in this issue, we know that starting treatment as quickly as possible is critical to protecting the brain, slowing disease progression and preventing disability.







